William Tang
McGill MSc Epidemiology
Waterloo BSc Health Science
Project Management Professional
Personal Details
- Phone: 1-548-328-4611
- EMAIL: william.tang1013@mail.mcgill.ca
- Birthday: October 13
- Food: Dim Sum
- Sport: Badminton
Public Health Journey
I am a Scientist by training and a Project Manager by trade.
Over the past few years, I have led and supported health research across Alberta, Ontario, and Quebec. These diverse experiences revealed a critical disconnect in our industry: Great science often stalls due to poor execution.
My career aims to solve this problem.
The Foundation (BSc & Co-op): The University of Waterloo laid the scientific foundation of my career. The undergraduate coursework provided diverse exposure to various aspects of health research, combined with 2 years of work experience through the co-op program. This allowed me to apply theories from the lecture hall directly to our healthcare system.
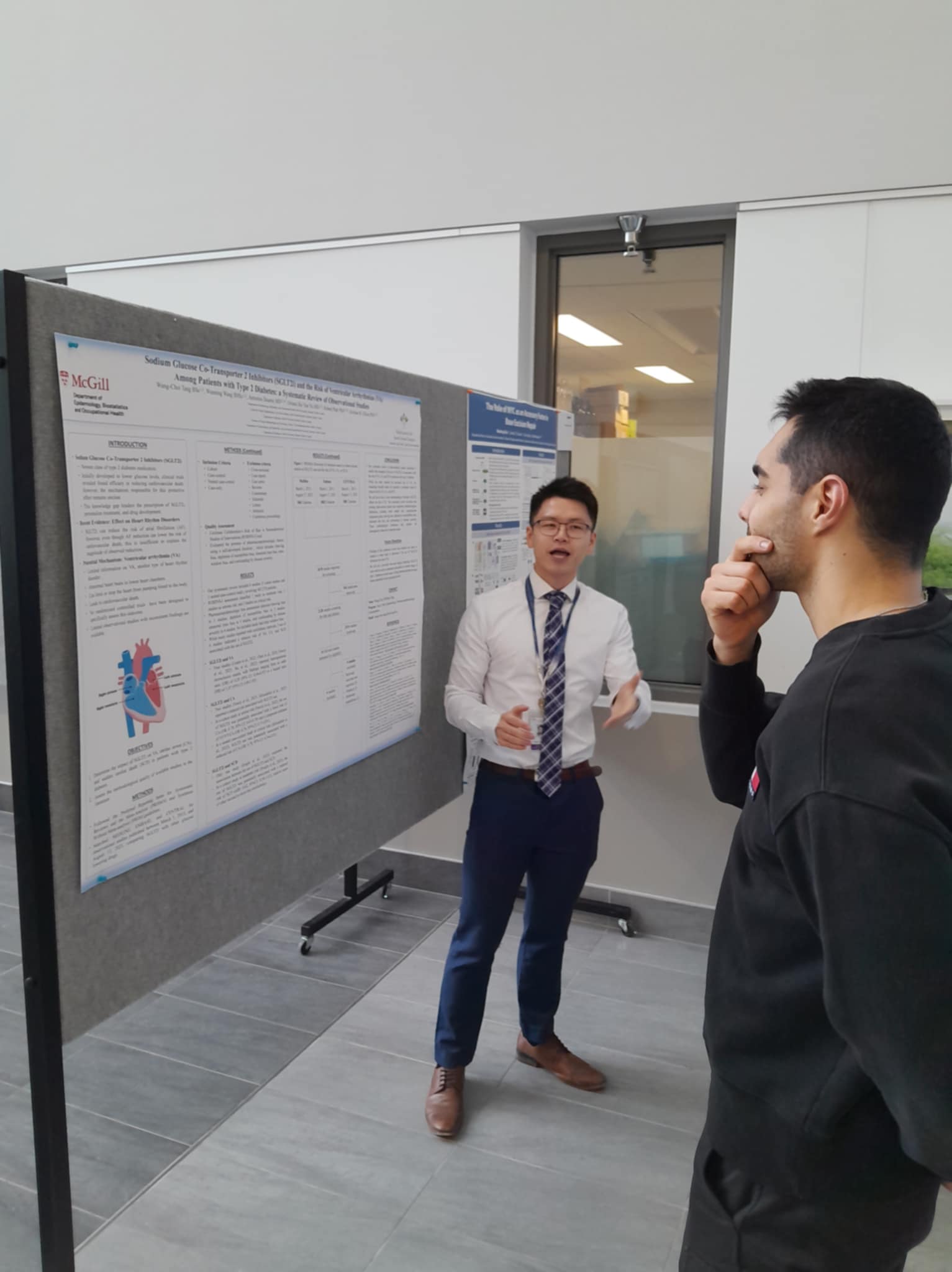
The Scientist (MSc): My diverse work experiences inspired me to pursue a Master’s in Epidemiology at McGill University. There, I received rigorous training in study design and causal inference from some of the brightest minds in the world. My work centered on leading Real-World Evidence (RWE) studies, evaluating the post-market cardiovascular safety of diabetes medications.
The Project Manager (PMP): Throughout my career, I have noticed that Scientists often struggle with operations and Project Managers usually lack the scientific foundation. This insight inspired me to pursue the Project Management Professional (PMP) certification. I now integrate project management best practices into the research lifecycle to ensure studies deliver maximum value.
The Hybrid Approach: I operate at the intersection of these two worlds. I can design protocols and manage the execution. My goal is to ensure that operational bottlenecks never get in the way of scientific discovery.
Let’s Connect: I love meeting new people. Feel free to send me an email. I am always happy to connect.
Expertise
Real-World Evidence (RWE) Generation
Strategy & Execution: Transformation of administrative and clinical data into decision-grade evidence. Generation of actionable insights that inform clinical practice and health policy.
Research Operations & Performance Monitoring
System Oversight: Development of centralized performance frameworks (KPIs) to track budget utilization and resource allocation. Transformation of manual inefficiencies into automated reporting ecosystems.
Study Design & Feasibility Analysis
Risk Management: Integration of project management frameworks into protocol development to ensure operational feasibility. Anticipation and mitigation of bottlenecks prior to study launch.
Statistical Analysis & Data Interpretation
Technical Translation: Bridge between complex statistical modeling and plain-language summaries. Delivery of clear, data-driven narratives for non-technical stakeholders and sponsors
Pharmacoepidemiology & Safety Surveillance
Safety Evaluation: Assessment of post-market safety and medication effectiveness. Mitigation of methodological challenges including confounding and selection bias, to ensure evidence validity
Change Management & Process Optimization
Adoption Strategy: Oversight of technology implementation and Standard Operating Procedures (SOPs). Facilitation of team buy-in ensuring operational continuity across research workflows.
Systematic Reviews & Meta-Analysis
Evidence Synthesis: Identification of knowledge gaps through rigorous literature appraisal. Establishment of scientific rationale to ground new research in the current global landscape.
Regulatory Compliance & Ethics
Audit Readiness: Full adherence to regulatory and ethical (REB) standards. Assurance of compliance at every stage of the project lifecycle to mitigate legal and reputational risk
Knowledge Translation & Stakeholder Engagement
Strategic Communication: Production of decision-grade reports for clinicians and policymakers. Alignment of diverse stakeholder expectations to sustain long-term collaboration..
Education
Project Management Institute
Project Management Professional Certification
Certification Status: Active
McGill University
Master of Science in Epidemiology
Pharmacoepidemiology Specialization
University of Waterloo
Bachelor of Science in Health Science
Health Research Specialization
TRAINING
- HLTH 173: Indigenous Health
- HLTH 230: Introduction to Health Informatics
- HLTH 260: Social Determinants of Health
- HLTH 280: Applied Public Health Ethics
- HLTH 290: Introduction to Health Neuroscience
- HLTH 340: Environmental Toxicology & Public Health
- HLTH 341: Principles of Pathobiology
- HLTH 350: Principles of Occupational Health
- HLTH 370: Ecological Determinants of Health
- HLTH 448: Advanced Social Determinants of Health
- PPHS 602: Foundation of Population Health
- Undergraduate Thesis: Latent Classification of Patients with Schizophrenia
- Data Source: interRAI & OMHRS (Ontario Data Set)
- Master’s Thesis: Sodium Glucose Co-Transporter 2 Inhibitor and the Risk of Ventricular Arrhythmia
- Data Source: CPRD Aurum (United Kingdom Data Set)